Quantum Mechanics: A Deep Dive into the Nature of Reality.
Quantum mechanics (QM), also known as quantum physics or quantum theory, is the branch of physics that deals with the behavior of matter and energy on extremely small scales—such as that of atoms and subatomic particles. It is a theory that explains phenomena that cannot be described by classical physics, especially when objects are at microscopic scales, such as electrons, photons, and other elementary particles.
Quantum mechanics fundamentally challenges our classical understanding of the world. Classical physics, particularly Newtonian mechanics and electromagnetism, works incredibly well for macroscopic systems (like the motion of planets or the behavior of everyday objects). However, when it comes to the behavior of tiny particles, the rules that govern the macroscopic world simply do not apply. Quantum mechanics provides a mathematical framework that accurately describes the behavior of matter and energy at these microscopic scales.
1. Wave-Particle Duality
One of the first major discoveries that led to the development of quantum mechanics was the concept of wave-particle duality. This refers to the observation that particles, like electrons and photons, can exhibit both particle-like and wave-like behaviors depending on the experimental setup.
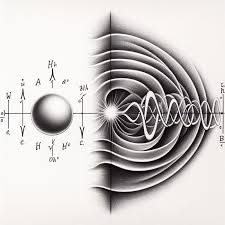
Particle Nature:
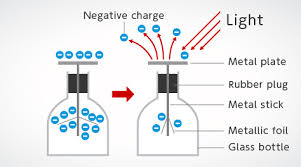
Light, for instance, was once thought to be purely a wave, but experiments, like the photoelectric effect (explained by Albert Einstein in 1905), demonstrated that light can also behave as a stream of particles called photons. In the photoelectric effect, light shining on a metal surface can cause electrons to be ejected from the surface, but only if the light has a frequency above a certain threshold. The result could not be explained if light were purely a wave, but works perfectly when considering light as consisting of discrete packets of energy, or photons.
Wave Nature: On the other hand, electrons and other particles, which were once considered purely particles, can also exhibit wave-like properties. This was demonstrated in the famous double-slit experiment. When a beam of electrons is directed at a barrier with two slits, the electrons do not behave like individual particles. Instead, they interfere with themselves as though they are waves, creating an interference pattern on a screen behind the barrier. This wave-particle duality is now considered a cornerstone of quantum mechanics.
2. The Uncertainty Principle
One of the most profound and puzzling aspects of quantum mechanics is the Heisenberg Uncertainty Principle, formulated by Werner Heisenberg in 1927. This principle states that it is impossible to simultaneously know both the position and the momentum of a particle with perfect accuracy.
The more precisely we know a particle’s position, the less precisely we can know its momentum, and vice versa. This is not due to limitations in measurement tools but is an inherent property of quantum systems. This implies that there are fundamental limits to our knowledge of the state of a system.
In practical terms, this uncertainty means that particles do not have definite positions and velocities at any given moment in time in the way classical particles do. Instead, quantum systems are described in terms of probabilities. For example, an electron in an atom doesn’t have a definite position but instead exists as a wavefunction that describes a probability distribution of where it might be found.
3. Superposition and Entanglement
Two other core principles of quantum mechanics are superposition and entanglement, which challenge classical notions of reality.
Superposition refers to the ability of quantum systems to exist in multiple states at once. A classic example is Schrödinger's thought experiment involving a cat in a box. According to quantum theory, until the box is opened and the cat is observed, the cat is in a superposition of being both alive and dead simultaneously. This is because the quantum particles that make up the cat’s body (and everything else in the box) exist in multiple states simultaneously, with each state corresponding to a different outcome (alive or dead).
Entanglement is a phenomenon that occurs when two or more particles become correlated in such a way that their states are interdependent, regardless of the distance between them. This means that measuring one particle will instantly determine the state of the other, no matter how far apart the particles are. Albert Einstein famously called this "spooky action at a distance." Entanglement has been experimentally verified and is a key feature of many emerging technologies, such as quantum computing and quantum cryptography.
4. The Schrödinger Equation
The Schrödinger equation is the mathematical foundation of quantum mechanics. It describes how the quantum state of a physical system changes over time. The equation, developed by physicist Erwin Schrödinger in 1926, is analogous to Newton's second law in classical mechanics, but instead of describing the motion of macroscopic objects, it describes the evolution of the wavefunction of a quantum system.
The wavefunction is a complex mathematical function that encodes all the information about a quantum system. The square of the wavefunction’s amplitude at a given point represents the probability of finding a particle at that point in space. The Schrödinger equation provides the means to calculate the wavefunction and determine how it evolves over time, thus predicting the behavior of quantum systems.
There are two forms of the Schrödinger equation: the time-dependent equation, which is used for systems where the quantum state changes over time, and the time-independent equation, which is used for systems in which the quantum state does not change over time (such as particles in a stable potential like electrons in an atom).
5. Quantum Tunneling
Quantum tunneling is a phenomenon where particles pass through energy barriers that they classically should not be able to cross. In classical physics, if a particle does not have enough energy to surmount a barrier, it will simply bounce back. However, in quantum mechanics, the wavefunction of a particle extends beyond the barrier, and there is a non-zero probability that the particle will "tunnel" through the barrier and emerge on the other side.
This effect has been crucial in various fields, including nuclear fusion (where particles in the Sun’s core tunnel through energy barriers to fuse and release energy) and semiconductor technology (where tunneling is an important factor in the operation of certain types of transistors).
6. Quantum Field Theory (QFT)
Quantum Field Theory is a more advanced framework that combines quantum mechanics with special relativity and describes how fundamental particles interact with each other and with fields. According to QFT, the particles we observe are excitations or disturbances in underlying quantum fields that permeate all of space.
The most well-known QFT is the Standard Model of particle physics, which describes the electromagnetic, weak, and strong nuclear forces, as well as the fundamental particles that make up matter (quarks, leptons, and bosons). The Higgs boson, for example, is a quantum excitation of the Higgs field and was experimentally discovered in 2012 at CERN.
QFT has been highly successful in explaining particle interactions, but it is still incomplete. It does not incorporate gravity, which remains the domain of general relativity, and one of the greatest challenges in modern physics is to reconcile quantum mechanics with general relativity to form a Theory of Quantum Gravity.
7. Quantum Computing and Technology
One of the most exciting applications of quantum mechanics is in the realm of quantum computing. Classical computers use bits, which can be either 0 or 1, to process information. In contrast, quantum computers use qubits (quantum bits), which can be both 0 and 1 at the same time due to superposition. This allows quantum computers to process exponentially more data in parallel compared to classical computers.
Quantum entanglement is also used in quantum computing to create interdependent qubits, further enhancing the power of quantum algorithms. Algorithms like Shor’s algorithm (for factoring large numbers) and Grover’s algorithm (for searching unsorted databases) demonstrate the potential of quantum computers to outperform classical ones in specific tasks.
Quantum cryptography, based on principles like entanglement and the no-cloning theorem (which says that quantum information cannot be copied), is already being used to develop highly secure communication systems that are theoretically immune to eavesdropping.
Conclusion.
Quantum mechanics represents one of the most successful and mind-bending theories in the history of science. It has not only revolutionized our understanding of the microscopic world but also paved the way for groundbreaking technologies such as semiconductors, lasers, and quantum computers. However, many aspects of quantum mechanics remain counterintuitive and challenge our classical notions of reality.
The theory continues to evolve, and many open questions remain, particularly in trying to unify it with the theory of gravity. Despite its abstract nature and challenging mathematical formulations, quantum mechanics is one of the cornerstones of modern science and continues to drive both theoretical and applied research in physics, chemistry, and technology.


You must be logged in to post a comment.